Biology
Cell Biology
A branch of biology that studies the structure, corresponding function and subsequent behaviour of components within a cell.
Biochemistry
Solving biological issues with chemical perspectives and techniques.Focusing on the intracellular entities, treating them as chemical blocks and studying the their functionality thus map out the landscape about how life works.
Molecular Biology
Molecular biology studies the composition, structure, function and behaviour of bio-active and/or bio-significant molecules, such as nucleic acids and proteins.
Genetics
Study heredity in the perspective of elemental blocks from DNA and their temporal/spatial distribution/variation in organism. Originality of diseases (abnormality) and driving force of evolution could be derived from thorough understanding of genetics.
X-omics
Source of large-scale and comprehensive biological data assembled from Genomics Transcriptomics, Proteomics, Metabonomics, Microbiomics.
Systems biology
Analysis and modeling of complex biological systems based on data acquired by X-omics.
Synthetic biology
Design of new device and circuits based on biological components.
Epidemiology
Distribution and determinants of disease in population.
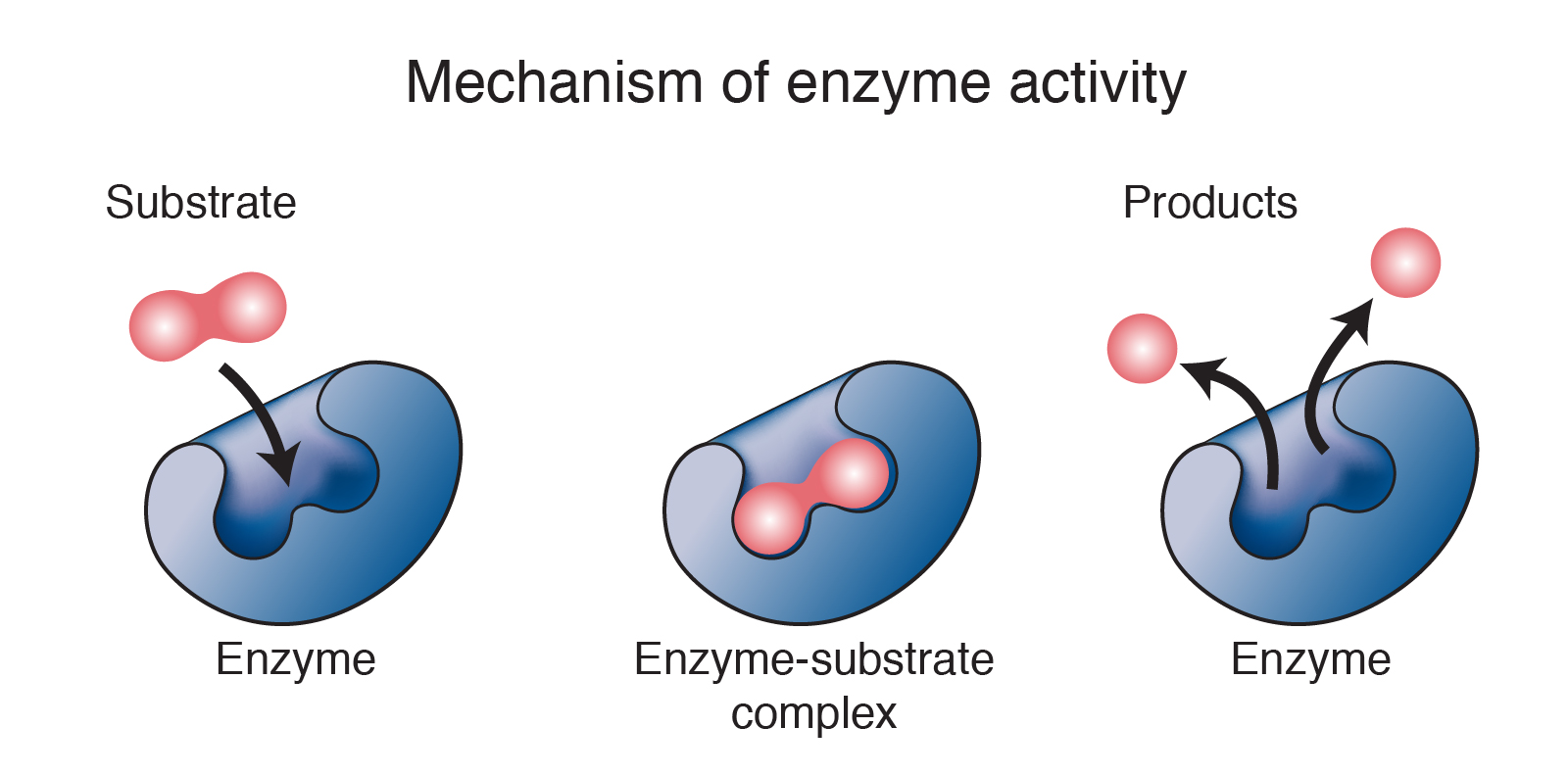
Enzyme
An enzyme is a biological catalyst that is capable of accelerating a specific chemical reaction in cells. The enzyme is not destroyed during the reaction process and could be used again and again (under the sustained condition). In most cases, enzymes are proteins.
Phase I/II biotransformation
metabolism of a drug can be divided into 2 phases. Phase I mainly involves the breakdown (mainly by hydrolysis and oxidation). Phase II mainly involves the conjugation of chemical groups (polar in most cases) to make drug more soluble and suitable for excretion.
Cytochrome P450
A family of key enzymes contain heme as the cofactor to function as mono-oxygenases. It is the typical phase I drug metabolizing enzyme and are involved in so many components’ metabolism from drug and food. They can be easily induced and inhibited by their substrate thus have a outstanding role when studying the drug-drug interaction (DDI). e.g. Patients who are taking Alvastatin are not allowed to eat grapefruit.
Drug targets
Molecules that are intrinsically associated with particular diseases and could be specifically addressed by a drug to take action. Most of the known drug targets are proteins.
Active site
Catalytic center of enzymes that bind substrate(s) and initiate reactions. For enzymes that are proteins, side chains along the backbone of key amino acids constructing the active site, shape it into specific size with specific chemical behavior.
Cofactor/Prosthetic group/Coenzyme
Cofactors are necessary non-peptide components required for enzymes to function properly. Cofactors can either be inorganic metal ions or organic molecules. The assistance of cofactors for enzyme function is achieved by binding to the inactive form of enzyme (apo-enzyme) to produce the catalytic active form (holo-enzyme). A prosthetic group is a type of cofactor that tightly bind to the assisted enzyme and is not easily to be removed. A coenzyme is a specific type of cofactor as they are organic small molecules.
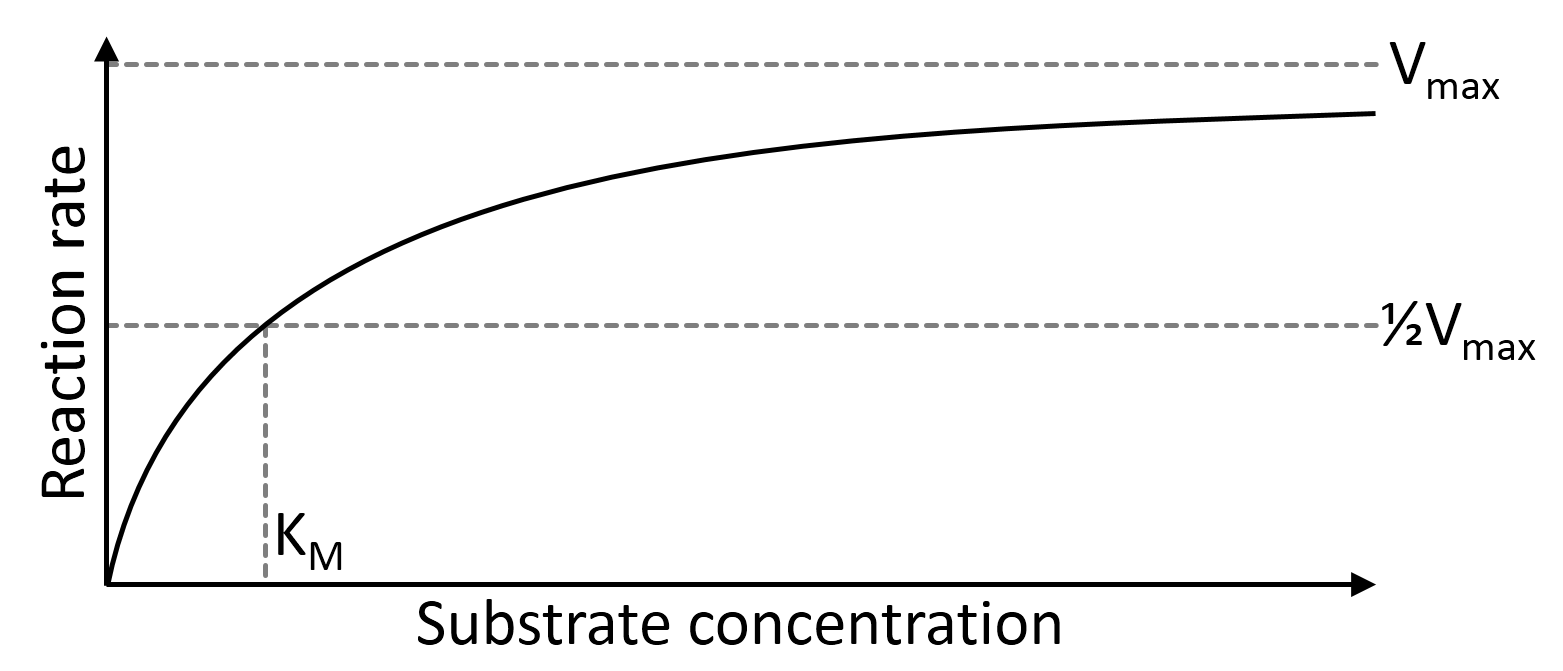
Michaelis-Menten equation
Michaelis–Menten kinetics describe the typical kinetic behaviour of enzymes. The name was given after German biochemist Leonor Michaelis and Canadian physician Maud Menten. The Michaelis–Menten kinetics model describes the rate of enzymatic reactions \(v\) in the form of Michaelis–Menten equation showing bellow:
Here, enzyme reaction rate \(v\), the rate of forming product \([P]\), is related with substrate concentration \([S]\) \(V_{max}\) describes the maximum reaction rate achieved by the studied system. It would be reached when the substrate concentration is saturated under a given enzyme concentration. The Michaelis constant \(K_{M}\) is numerically equal to the substrate concentration where half \(V_{max}\) is reached. In most of the enzyme catalyzing single-substrate reactions, their kinetics behaviours are assumed to fit Michaelis-Menten equation, regardless of further assumptions.
Kinase
Types of enzyme responsible for substrate phosphorylation.
Receptor tyrosine kinase
Tyrosine kinase is a type of kinase for tyrosine phosphorylation. It functions as an “on” or “off” switch in many cellular signalling process. Receptor tyrosine kinase is a subclass of tyrosine kinase that serves as cell surface receptor with high-affinity for many polypeptide growth factors, cytokines, and hormones.
G protein coupled receptors (GPCR)
A large group of evolutionarily-related proteins serve as cell surface receptors to produce cellular response activation upon signal outside cell. The transmembrane domain of GPCRs pass through the cell membrane seven times (typical structure characteristics of GPCRs). Ligands can either bind at extracellular N-terminus and loops or within the transmembrane helices of GPCR. Effective binding of ligand would cause conformational change. Subsequent dissociation of \(\alpha\) subunit from the conjugated G-protein would further facilitate intracellular signal processing.
![Structural and conformational scheme of GPCR[1].](https://dp-public.oss-cn-beijing.aliyuncs.com/community/knowledge_base/schematic_GPCR.jpg)
Catalytic receptor
Type of cell surface protein with the ligand binding site localized at the extracellular surface of the plasma membrane and the functional region possessing catalytic activity on the intracellular face of the plasma membrane. The two parts are linked by a single transmembrane-spanning domain consisting of 20–25 hydrophobic amino acids. It commonly exists and functions as a dimer. Endogenous ligands for catalytic receptor are often peptides or proteins.
Transport protein
A transmembrane protein which function to allow selective passage of specific molecules from the external environment and is able to translocate ions, small molecules, or macromolecules. Transport proteins may be divided into subgroups as channels and carriers.
Carrier protein
Active carrier proteins function in the energy-consumed manner and are able to translocate the substances against concentration gradient. Passive carrier proteins assist the substance by facilitated diffusion.
Ion channel
An ion channel is a type of transmembrane protein that mediates the passage of ions through the membrane. The major differences between ion channels are ion carriers are: (1).high efficiency, usually \(10^6\) per second (or higher); (2).translocation of ions down their electrochemical gradient in an energy conservation way.
Nuclear hormone receptors (NHR)
A class of transcriptional factors to regulate gene expression regulated by their binding ligands. The ligand binding domain (LBD) is capable of recognizing specific ligands to stimulate conformational change (dimerization) of NHR. The DNA binding domain (DBD) mediates the receptor towards its hormone response elements (HRE). DBD functions in the form of a dimer with each monomer recognizing a six base pair sequence of the targeted DNA.
Ubiquitination
A biological process of protein degradation (intracellularly). The protein is first labelled with a ubiquitin, a 76-amino-acid protein, through a three-step process with help of ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2), and ubiquitin-protein ligase (E3), facilitating mono-ubiquitination. The labelled ubiquitin chain could be extended by adding more ubiquitin, resulting in polyubiquitination. The 26S proteasome recognizes the polyubiquitination as a signal to initiate proteolysis and process the protein for degradation.
Proteasome
Huge protein complex to break peptide bonds for unneeded or damaged proteins.
Heat shock proteins (HSP)
Molecular chaperones (proteins) to assist protein functioning in response to stressful conditions (eg. exposure to cold and/or UV light, wound healing etc). HSPs are named according to their molecular weight. HSP90 refers to HSPs which are 90 kilodaltons in size. Ubiquitin (8 kilodaltons) also possess heat shock protein features.
Tubulins
Structural unit for living cell skeletal system. Tubulins are proteins that can be polymerized into long chains or filaments to assemble into microtubules - hollow fibers that serve as cell skeletal system.
Binding Site Detection for Receptors
Not all functional components in our body can be drug targets. However, this doesn’t mean they cannot be modulated. Sometimes they are just too hard to be accessed accurately due to their distribution in tissue or a structural factor, while in other cases inhibition of these components cannot trigger the expected downstream reaction due to intrinsic homeostasis / ignorance of its mechanism. In most cases, orthosteric binding sites ( the pocket to binding endogenous ligand) can be easily determined by sequence / structure alignment. These site may lack selectivity, rendering growing interest in allosteric site detection. (sites not directly binding the endogenous ligand, but modulate its binding behavior) Traditional methods for allosteric site detection rely on MD simulation. See: Investigating Cryptic Binding Sites by Molecular Dynamics Simulations
Orthosteric/Allosteric Regulation A protein can have endogenous ligands and protein-protein binding partners. If a drug binds the protein in areas directly involved in endogenous binding, its effect on the protein is called orthosteric regulation. If the drug binds other areas (far away) but can affect the behavior in this area, its effect on the protein is called allosteric regulation. Orthosteric regulation is easier to study: such binding can at least compete with endogenous partner, affecting target behavior. Allosteric regulation is much harder to research, requiring dynamic insight to determine the relationship between orthosteric site and the potential allosteric site.
Covalent Regulation Traditionally, a drug molecule binds to the target without a reaction with it. It can bind and dissociate, resulting in a chemical equilibrium. However, some novel types of drugs try to form a chemical bond with the target, binding to them permanently. Giving the obvious Sequelae effect (the drug effect can maintain a long time after the drug’s blood concentration becomes low), this kind of regulation can be both effective and risky.
Immunology
A branch of physiology raising huge interest recently; studies the immune system of human body.
Lymphocyte A type of white blood cell that plays a vital role in immune responses. There two types of lymphocyte: B-cells and T-cells.
B-cells and T-cells B-cells are a type of lymphocyte that are able to produce antibodies. T-cells are involved in cell-killing (directly kill the virus-infected cells), immune response amplification (via cytokines, a signal protein secreted from T-cells) and cell memory that enable an organism to respond to the same infection more quickly and efficiently if infection happen again.
Antigen and antibody The term antigen originally referred to a substance that may trigger an immune response and serves as a antibody generator. Antibodies (or immunoglobulins) are large, Y-shaped protein secreted from B-cells to recognize and neutralize antigens.
Complement system The complement system functions via the cascade involving distinct plasma proteins that react with one another to opsonize pathogens and induce a series of inflammatory responses to fight infection. It works as enhancing and or complementing the effects of antibody activity and is firstly evolved as part of the innate immune system.
Cluster of differentiation antigen (CD) Surface proteins on leukocytes, reflecting differentiation stage or activation state of the cell and can be recognized by specific monoclonal antibodies.
Epitope Epitope is the antigenic determinant lying on the antigens to simulate immune responses. Binding and subsequent reaction of immune cells and antibodies with antigens is initiated via the recognition of epitope.
Antigen-presenting cell (APC), Major histocompatibility complex (MHC) and Human leukocyte antigen (HLA) APCs are cells possessing the ability to present an antigen for T-cell recognition. The heterogeneous group (protein complex) on the APC surface for antigen presentation is called major histocompatibility complex (MHC). There are two type of MHC, class I and class II, differed by structure and expressed cell types. MHC in human is also called human leukocyte antigen (HLA). There is significant work aiming to solve the recognition pattern issues of MHC with presented antigen. AI models have achieved rather ideal accuracy for the prediction task to define whether an antigen (mainly short peptide sequence) could be presented by MHC (thus stimulate the immune reaction from T-cells with much possibility) to design more efficient immune regulators (neoantigen).
Cytokines Cytokines are messenger proteins released from immune cells to regulate immune responses. Abnormal activities of cytokines could induce “cytokine storm” which has lethal impact.
Antigenicity and immunogenicity
When a foreign material (antigen) enters, an organism would initiate a barrier system to fight against and eventually eliminate this intruder. Antigenicity describes the ability of an antigen bind to, or interact with the products of the final cell-mediated response (such as B-cell or T-cell receptors). Immunogenicity measures the ability of the antigen to activate the immune response (including innate immune response and the subsequent adaptive (acquired) immune response). Immunogens possess antigenicty, while antigens may not always have immunogenicity. Metal ions are typically haptens, which are antigens, but would not trigger immune responses.
Monoclonal antibody, vaccine and neoantigen
Monoclonal antibodies are engineered antibodies that typically recognize the same epitope, and thus possesses high specificity towards the targeted antigen. Vaccines are the biological preparation containing an agent to initiate the immune responses to form a barrier thus protect the body from certain disease derived from infection. The agent of vaccine resembles the disease-causing microorganism and is often made from weakened or killed forms of the microbe, its toxins, or the surface proteins. Neoantigens are the translation product (protein) of mutated DNA in cancer cells. They are different from the original protein under physiological condition and may thus play a significant role in stimulating immune response against cancer cells.
Prediction and design of protein-protein interaction
Protein-protein interaction (PPI) is the basis for many biological processes to function properly. Specific recognition between the interacting proteins is established on the basis of physical contacts. The forces driving stable/favourable interaction come from electrostatic interaction, hydrogen bonding and/or the hydrophobic effects etc. Based on the forces performed by atom/atom groups, there exist recognition patterns in the aspects of protein sequence as conserved region formed by amino acids that possess similar physicochemical properties have been observed in certain type of PPI. With the understanding of the interaction forces and their corresponding protein sequences, recognition/interaction patterns of PPIs should be reasonably summarized in relation with their biological outcomes. These summarized patterns in forms of models could be further applied for biological effect prediction with the protein sequences as input. Further, one could design functional protein sequences to achieve the desired bio-activity.
Yield, Solubility, Stability of therapeutic Macromolecules
Therapeutic macromolecules are compounds with large molecular weight possessing therapeutics effects and are typically derived from biological processes. The commonly applied therapeutic macromolecules (macromolecular drugs) include peptides, proteins, antibodies, polysaccharides and nucleic acids. Procedures to collect therapeutic macromolecules include bio-synthesis, recombinant protein expression, conjugation and modification etc. In comparison with small-molecule drugs, stable pipeline construction to yield therapeutic macromolecules requires much more effort. It is also worth noting that, as therapeutic macromolecules are typically derived from biological process in organism, some of them possess favourable intrinsic properties such as being lipophilic/hydrophobic and many of them would be easily degraded when recognized by cell metabolizing systems. Thus, enhancing the solubility of therapeutic macromolecules to facilitate desirable distribution properties as well as to sustain the intact entities thus gain stability to occupy therapeutic window wide enough to take action are another important issues for future discovery.
Immuno-therapy
Immuno-therapy functions by activating/mediating/enhancing immune responses of the patients to fight against diseases (cancer).
Cell therapy
Engineered cells (isolated from patients) with therapeutic effects are injected/grafted/implanted (back) into the patient’s body to treat the disease. The most well known cell therapy is CAR-T, where chimeric antigen receptor T cells are genetically engineered to produce an artificial T cell receptor to take action in the way of immunotherapy.